
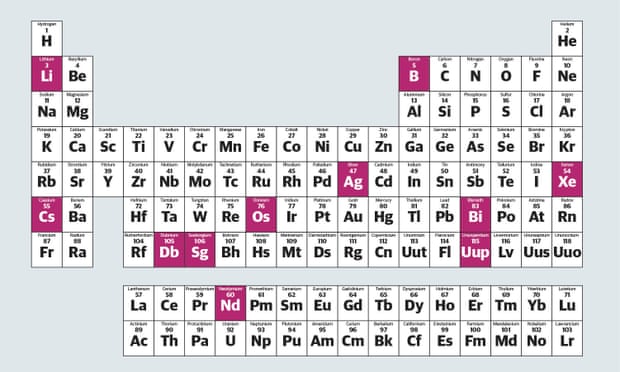
At last, the periodic table looks tidy. To nobody’s surprise, the discovery – and indeed creation – of a few atoms of elements number 113, 115, 117 and 118 has now been verified by the International Union of Pure and Applied Chemistry (IUPAC), and the seventh period is complete, giving the main table a nice, smooth bottom row. As soon as the new elements are officially named, the world’s chemistry textbooks can be reprinted with what may well be the final version. (There might be an eighth period out there too, but let’s not think about that for now.)
Since we first began to understand that each element consists of a specific number of protons surrounded by the same number of electrons (and flavoured with various numbers of neutrons), it has been easy to speculate about finding more. The Russian chemist Dmitri Mendeleev first arranged them in the now familiar way in 1869, having seen the table in a dream. Henceforth, if anyone asks you what the universe is, you can give (as a short answer): “This.” So here are some of the most intriguing elements on the all-singing, all-dancing new periodic table.
3. Lithium
Very weird stuff, lithium. At room temperature, it’s a solid metal that floats on water. If people with bipolar mood disorder swallow lithium compounds, it seems to smooth out their highs and lows. It is also responsible for the smartphone revolution (and perhaps an electric car one to follow), by making it possible to build light, powerful batteries. Much of the world’s available lithium is found in Bolivia and Peru, so it may yet turn the Andes into the new Middle East.
5. Boron
If you want to feel sorry for an element, go for boron. Numbers one and two (hydrogen and helium) make up stars. Three and four treat mental illness (lithium) and build rockets (beryllium). Six, seven and eight (carbon, nitrogen, oxygen) are the basic constituents of life. And there in the middle, at number five, sits boron, the stuff that makes silly putty soft yet bouncy.
54. Xenon
A fairly cool element, it must be said. It certainly has the coolest name, and like the other noble gases, helium, neon, argon, krypton (nothing to do with Superman) and radon, it just floats around not doing much. Under extreme pressure, however, xenon can be forced to make ultra-bright lights for massive cinemas.
83. Bismuth
The hipster element. No one’s heard of bismuth. Those who have, though, think it’s the last stable element before everything becomes radioactive (ie, spontaneously breaks itself), but in fact it is just very, very, very, very un-radioactive. Generally, elements should behave a bit like the others around them, but bismuth doesn’t at all. Lead is poisonous, polonium is what killed Alexander Litvinenko and bismuth is the main ingredient in the stomach-settler Pepto-Bismol.

115. Ununpentium
Element number 115 will only be called ununpentium until its Russian discoverers come up with a better name. However, it has already been credited with some extremely far-fetched properties. According to Bob Lazar, a man who claimed to have worked on analysing alien technology in Area 51, ununpentium is the fuel used in alien spacecraft. It isn’t.
76. Osmium
When children ask you, “What’s the heaviest naturally occurring element?”, after explaining that they mean “most dense”, give them the answer: “Osmium.” At this point, many smart-alec children will say, “Don’t you mean iridium?” “No,” you can then say patiently. “That’s a common misconception based on mistaken measurement in the past.” Then buy them Theodore Gray’s superb The Elements app for their next birthday.
106, 105. Seaborgium, dubnium
Cold-war squabbling will be frozen into the periodic table for ever thanks to the rivalry between researchers in the University of California, Berkeley, led by Glenn Seaborg, and those at the Joint Institute for Nuclear Research in Dubna, Russia. The two teams discovered many radioactive heavy elements at similar times, and in secret, making it difficult to say who got there first. In 1997, the IUPAC finally decided to give dubnium to one side and seaborgium to the other – the only element ever named after a living person.

60. Neodymium
Makes really, really strong little magnets like the ones children play with and must never, ever swallow. While we’re down here, why are these two rows of elements – the lanthanides and actinides – set apart from all the others? Chemists have a secret answer to that: they actually belong in periods six and seven, but if you put them there the table would be too wide to print comfortably. Proof that nothing is ever really tidy.
47. Silver
Not cool for the reasons you think, but very cool for other ones. Solid silver is a strange thing to cherish in the form of coins or jewellery because it reacts with oxygen in the air and goes dark. When clean, however, it is the very shiniest element of all. It also conducts electricity better than any other element. When clean.
55. Caesium
The main thing everyone knows about the metal caesium is that it is so reactive that it will explode if you drop it in a bowl of water. Should you have any lying around, you could also watch it melt on a warm day as the temperature passes 28C. When not performing party tricks, caesium regulates the measurement of time for the human race by having a hyperfine atomic structure that is useful in atomic clocks.
This article was amended on 7 January 2016 to correct the chemical symbol for Flerovium in the main illustration, which is Fl and not Fi as originally stated.
Original article and pictures take http://www.theguardian.com/science/2016/jan/04/new-periodic-table-explained site
Комментариев нет:
Отправить комментарий